Thursday, December 12, 2024 | 3:00 – 4:00 PM CET (Central European Time)
With an aging population and limited healthcare resources, the traditional approach to patient care follow-up is becoming unsustainable. The future lies in automation, where AI-powered solutions will play a critical role. By integrating secondary data, connected devices and electronic clinical outcome assessments (eCOAs), these technologies will revolutionize how patient care is monitored and managed in real-life settings. As these innovations take hold, clinical research must evolve to incorporate and adapt to these new methods.
During our webinar on December 12, 2024 with Dr. Alexandre Malouvier, Ph.D. (Senior Director Scientific Affairs & Digital Innovation, eClinical Development & Delivery at ICON), he shared his expertise on the rapidly shifting landscape of healthcare.
What we learned about:
- The healthcare consequences of an aging population and limited resources
- How AI solutions can support the automation of patient follow-ups
- How clinical research is evolving to follow these developments.
Meet Our Speakers

Dragan Mileski
Co-Founder and CTO | Climedo
Dragan, an IT specialist driven by entrepreneurship, co-founded Climedo in 2017 with a mission to enhance medical treatment using intelligent software solutions. As the Chief Technology Officer (CTO), he leads teams dedicated to developing cutting-edge software aimed at transforming post-market Real-World Evidence (RWE) studies, providing real-time transparency of outcomes and fostering engagement with healthcare professionals.

Dr. Alexandre Malouvier, Ph.D.
Senior Director Scientific Affairs & Digital Innovation, eClinical Development & Delivery at ICON
Alexandre is helping ICON lead the CRO market by developing future-ready technology. A specialist in digital endpoints and COAs, he has extensive experience with RWE and secondary patient data. As a member of ICON’s AI Centre of Excellence, he also coordinates clinical research conferences and teaches at Université of Paris Cité.
What was on the Agenda?
-
Intro (Dragan Mileski)
-
Clinical Research meets Real-World: AI, eCOA and Digital Endpoints (Alexandre Malouvier)
-
Discussion and networking (all)
- Wrap-up (Dragan Mileski)
Key Takeaways
INSIGHTS
Gain valuable insights into the opportunities of AI-driven automation.
BEST PRACTICES
Discover how clinical research can be used to align with technology advancements.
NETWORKING & DISCUSSION
Talk to other industry professionals and find out how they are tackling challenges similar to yours.
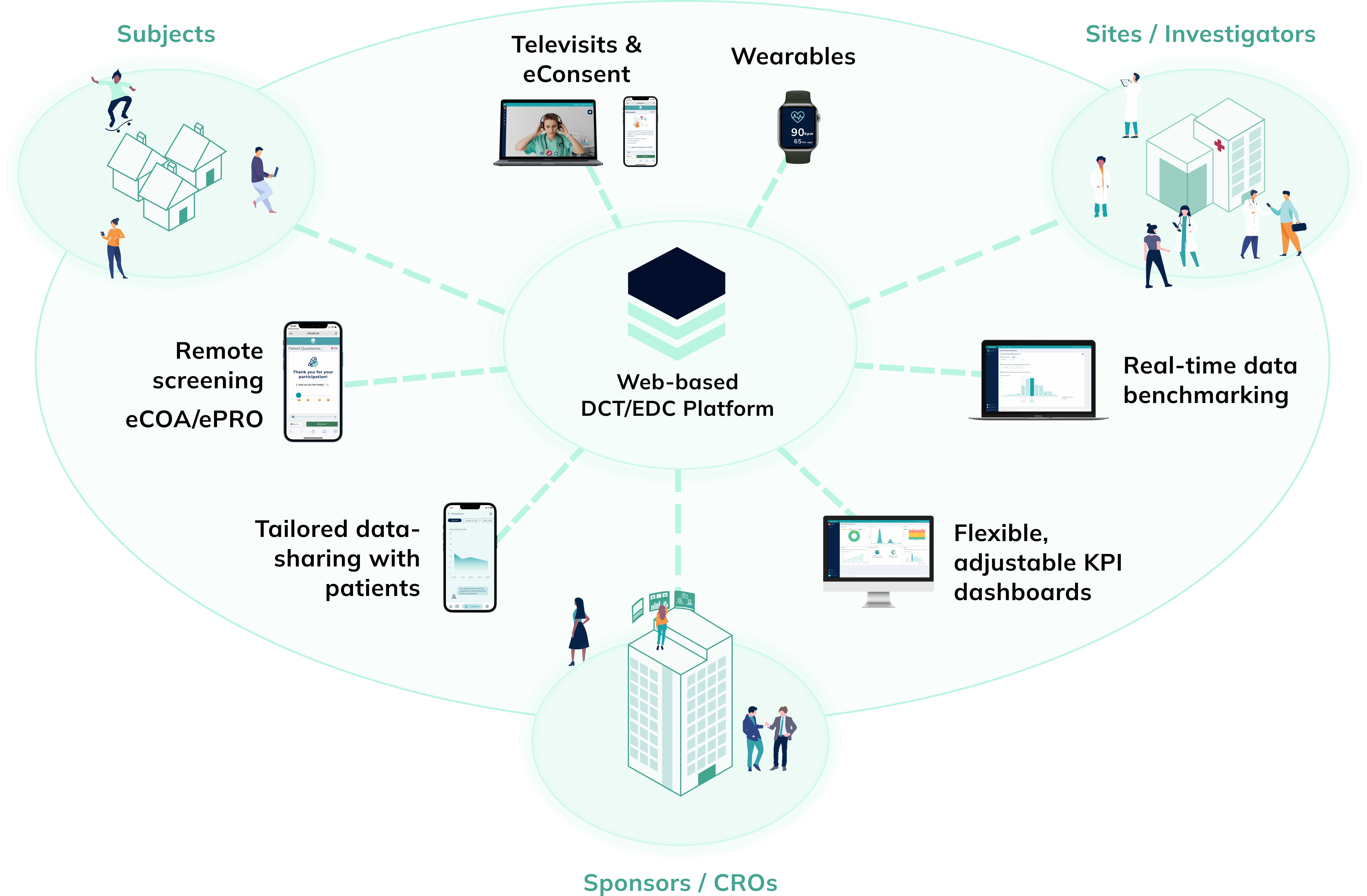
CLIMEDO
The leading European eCOA system for non-interventional studies, RWE and launch success
Climedo offers an all-in-one eCOA and EDC solution with hybrid capabilities for non-interventional studies and real-world evidence. By using a patient-centric approach and leveraging real-time data insights and visualizations around a study’s current progress, Climedo empowers its clients to better engage with healthcare professionals and other key opinion leaders (KOLs). This boosts awareness, stimulates scientific dialogue and accelerates the launch success of new medical innovations, thus reaching more patients faster.
Founded in Munich in 2017, Climedo is a leading trusted partner for pharma, MedTech, CROs and academia with over 1.7 million patients enrolled to date. Learn more at www.climedo.com.