In the fall of 2023, we collaborated with Siteworks Clinical Research to conduct a survey aimed at understanding trial physicians' perspectives on clinical trials and assessing the status quo of clinical research. Our survey involved 66 trial physicians across various therapeutic areas, predominantly based in Europe.
Respondents showed significant motivation to take part in trials, driven by their interest in advancing science, gaining early access to new treatments and improving their scientific reputation. However, they also identified challenges, including the considerable time commitment, difficulties in patient recruitment and shortages in staff. Moreover, respondents emphasized the importance of adequate remuneration, enhanced digital tools and realistic expectations regarding recruitment timelines from study sponsors and CROs. Among the digital solutions, eCRF, ePRO and EHR were highlighted as particularly promising and widely utilized. Interestingly, there was considerable interest among investigators in accessing treatment outcomes from anonymized data shared by other physicians, as well as a willingness to incorporate progression curves of patient parameters into their research. Despite this, the majority still favored personal monitoring over digital alternatives.
In summary, the report shows a need for fair remuneration, standardized digital technologies and less bureaucracy in clinical trials. Respondents also want more realistic timelines, particularly regarding recruitment.
Read the survey results and find out more about:
- Motivation and challenges to study participation for investigators
- Expectations of study sponsors and CROs
- Respondents' openness towards digital technologies in studies
We hope you enjoy reading the results and that they will provide you with valuable insights into the status quo of clinical research and give you new impetus for successful collaboration between investigators, CROs and study sponsors. If you have any questions, please do not hesitate to contact us: hello@climedo.de.
CLIMEDO
The leading European eCOA system for non-interventional studies, RWE and launch success
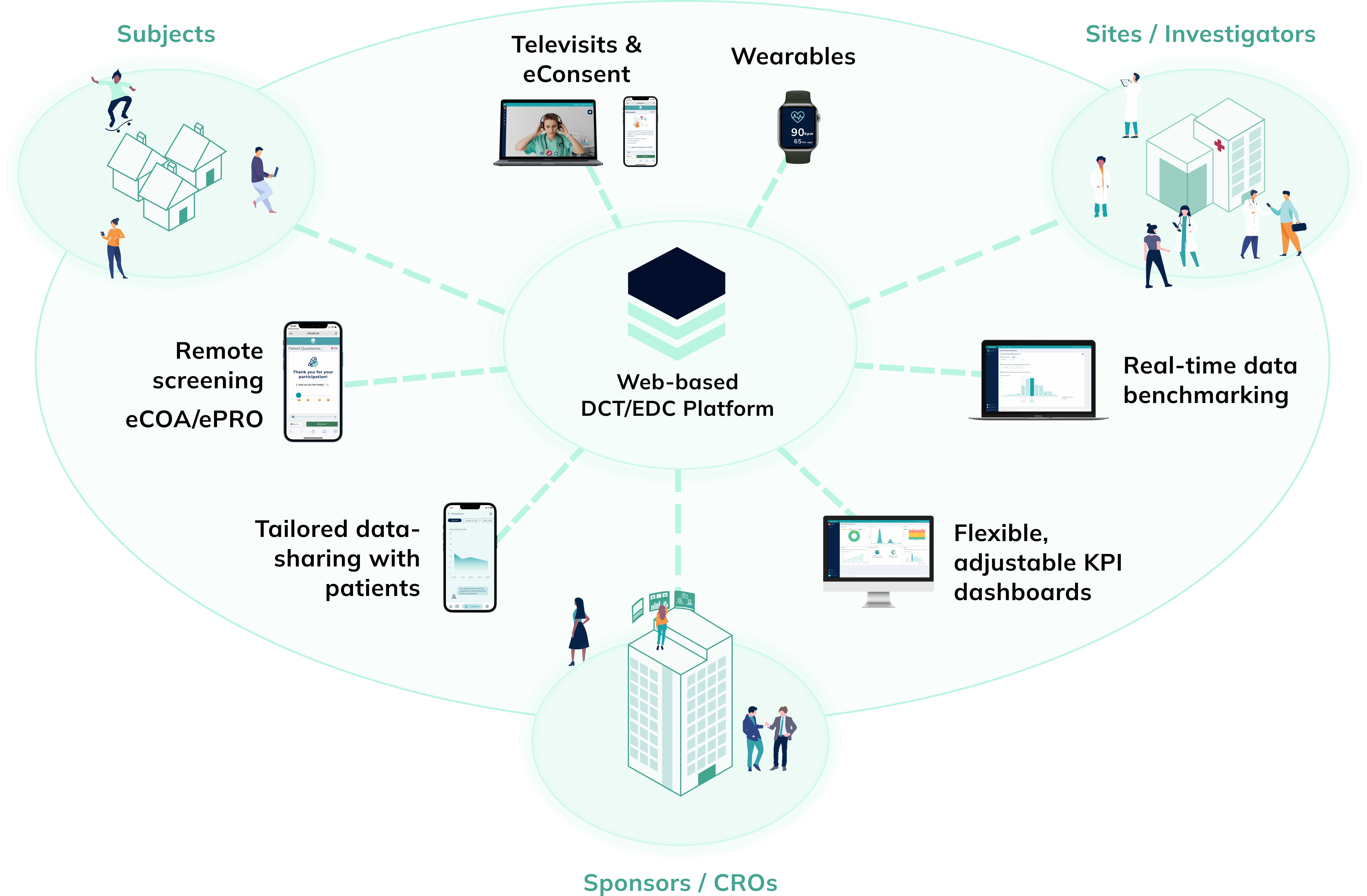
Climedo offers an all-in-one eCOA and EDC solution with hybrid capabilities for non-interventional studies and real-world evidence. By using a patient-centric approach and leveraging real-time data insights and visualizations around a study’s current progress, Climedo empowers its clients to better engage with healthcare professionals and other key opinion leaders (KOLs). This boosts awareness, stimulates scientific dialogue and accelerates the launch success of new medical innovations, thus reaching more patients faster. Founded in Munich in 2017, Climedo is a leading trusted partner for pharma, medtech, CROs and academia with over 1.7 million patients enrolled to date. Learn more at www.climedo.com.

SITEWORKS
We are a site network that conducts phase Ib to IV clinical trials for pharmaceutical products at multiple sites in Germany. Our study teams are active in various disciplines and settings (with and without standard care / outpatient and inpatient). The complexity of the challenges facing medical research institutions continues to grow and requires collaboration on strong partnerships to address these issues. With its extensive track record in this field, SITEWORKS acts as the link between sponsors or CROs, general practitioners or clinics and their patients. Our work is based on one key focus, namely on people.
At our sites we generate synergies between stringent quality requirements in the execution of clinical studies for pharmaceutical and medicinal products, the routine needed in medical healthcare, and satisfaction and confidence among our study participants.