Next Level of Hybrid Clinical Trials in Gastroenterology: Ask Us Anything
What’s it about?
Are decentralized clinical trials (DCTs) and hybrid trials just a temporary hype or here to stay? What are their true benefits and who is using them already?
In this pharma-exclusive session, we'll give you a quick overview about DCTs for gastroenterology and how they work. There will also be plenty of chance to ask our experts any specific questions you may have.
Exclusive Live Session for Pharma Professionals
Tuesday, September 20 2022
3 – 3:45 PM CET
Meet the experts
Join our experts Umberto and Matthias, and ask them any questions you may have. We look forward to seeing you there!
Want to have a free, personalized session just for you? No problem, email us your preferred time, date and topic at hello@climedo.de!
Dr. Matthias Roos
Climedo
Dr. Umberto Rosato
TFS – Clinical Contract Research Organization
What's in it for you?
COMPETITIVE ADVANTAGE
Find out why DCTs for gastroenterology, urology and gynecology matter and how your company can gain a competitive advantage with them.
BENCHMARKING
Learn how other companies are already mastering the power of DCTs, based on a European survey with over 100 respondents.
NETWORKING & DISCUSSION
Talk to other industry professionals in gastroenterology, urology and gynecology and find out how they are tackling challenges similar to yours.
Did you know?
80%
of patients are more likely to participate if they can use mobile technology.
Source: Global Virtual Clinical Trials Markets, 2021-2028
75%
of patients favor mobile trials over traditional ones.
Source: Global Virtual Clinical Trials Markets, 2021-2028
72%
of physicians report better or similar experiences with remote engagement.
Source: Sermo Covid-19 HCP Survey, 2020
Who is Climedo?
CLIMEDO
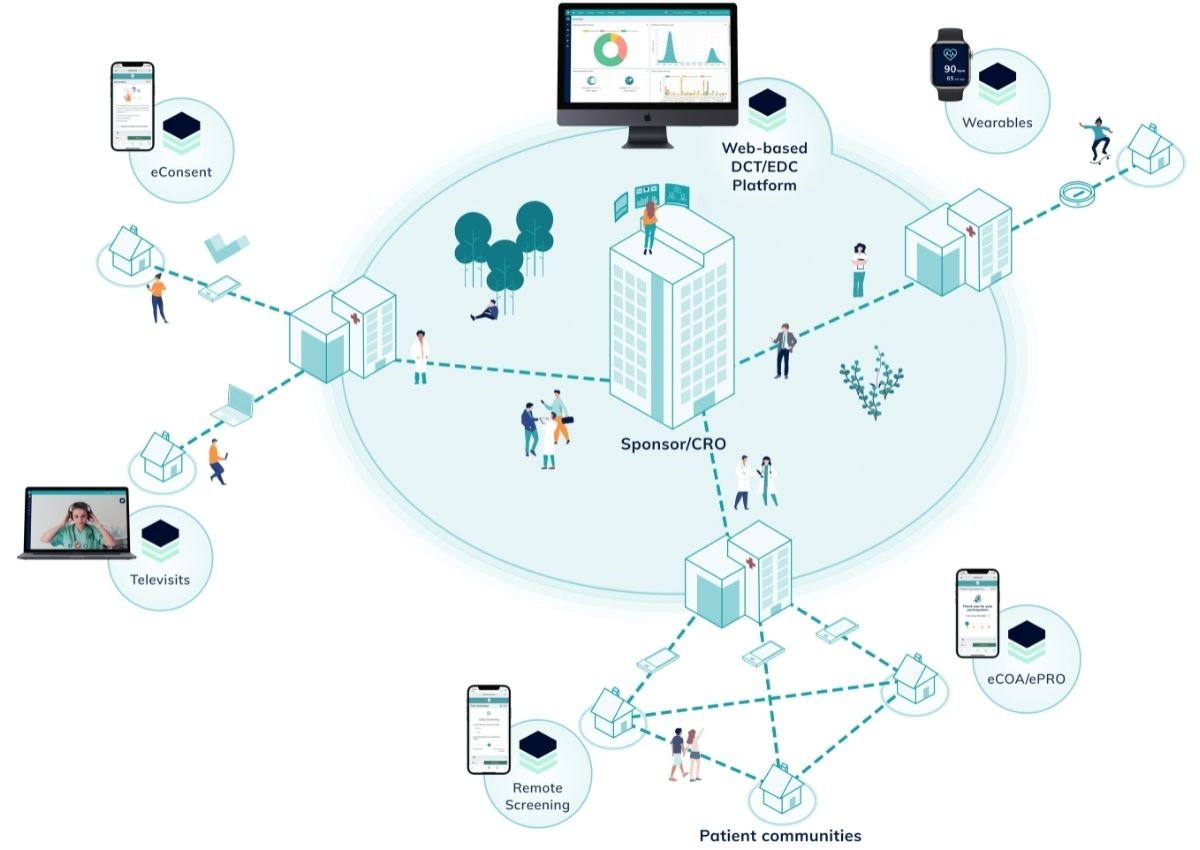
The Digital Platform for Innovative Clinical Trial Execution.
Our modular and user-friendly EDC solution ("Electronic Data Capture") enables pharmaceutical and medical device companies to efficiently validate their products and medical innovations in a virtual and patient-centric way.
By digitally connecting all parties involved, such as sponsors, doctors and patients, communication and data flow are simplified and accelerated significantly.