Cheat Sheet:
Clinical Trials for Rare Diseases – Overcoming Obstacles

Rare diseases can be very challenging for patients as well as the healthcare system. Access to treatment options is often very limited or doesn't exist. Mostly, clinical trials are the only way for patients to receive treatment. But how do you ensure a low drop-out rate, increased patient adherence and sufficient accessibility?
Our cheat sheet shows you which hurdles you have to overcome in clinical trials for rare diseases and how you can successfully master them, including:
- Accessibility
- Patient recruitment
- Patient adherence
- Taking medication on time
- Drop-out rates
We hope you enjoy this cheat sheet! If you have any questions or technical issues, please reach out to: hello@climedo.de.
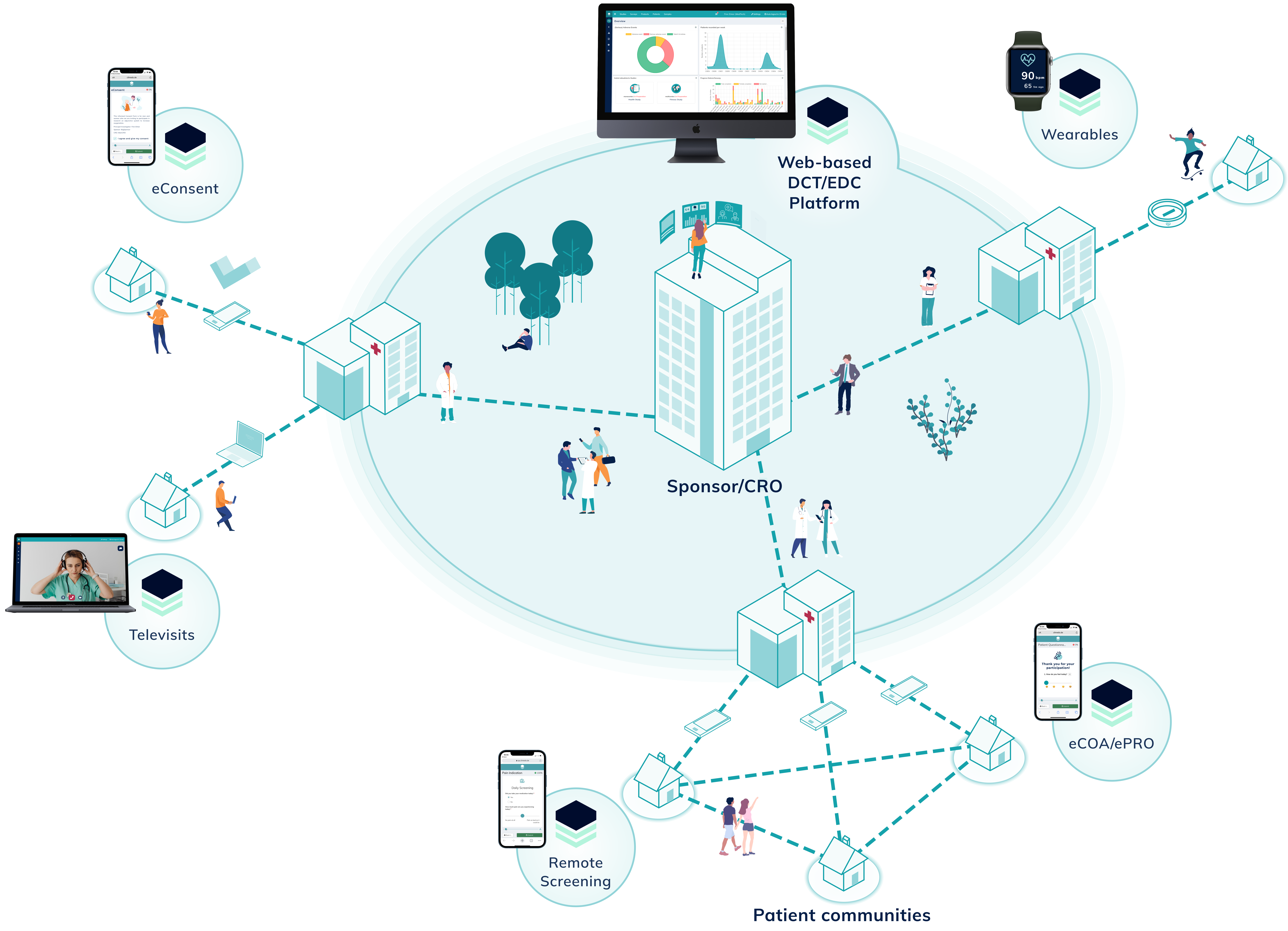
CLIMEDO
The Digital Platform for Innovative Clinical Trial Execution.
Our modular and user-friendly EDC solution ("Electronic Data Capture") enables pharmaceutical and medical device companies to efficiently validate their products and medical innovations in a virtual and patient-centric way.
By digitally connecting all parties involved, such as sponsors, doctors and patients, communication and data flow are simplified and accelerated significantly.