Whitepaper
Harnessing the Power of GenAI in RWE Studies
.png?width=1920&height=1280&name=Whitepaper%20Sept%201%20(1).png)
Generative Artificial Intelligence (GenAI) is transforming industries and healthcare is no exception. Its rapid adoption, evidenced by ChatGPT’s record-breaking growth to 200 million users in just two months, highlights its immense potential. In healthcare, GenAI is poised to revolutionize Real-World Evidence (RWE) studies, particularly in post-market clinical research.
As the healthcare sector embraces GenAI, however, it is crucial to implement best practices to ensure regulatory compliance and maintain data security. Prioritizing AI models trained on medical data, implementing robust quality assurance measures and adhering to ethical guidelines are vital steps in integrating GenAI responsibly.
Explore the following aspects in the Whitepaper:
- GenAI Fundamentals
- Promising Use Cases in Post-Market Studies
- Best Practices and Compliance
Download our free Whitepaper for valuable insights how GenAI can take your RWE studies to the next level.
CLIMEDO
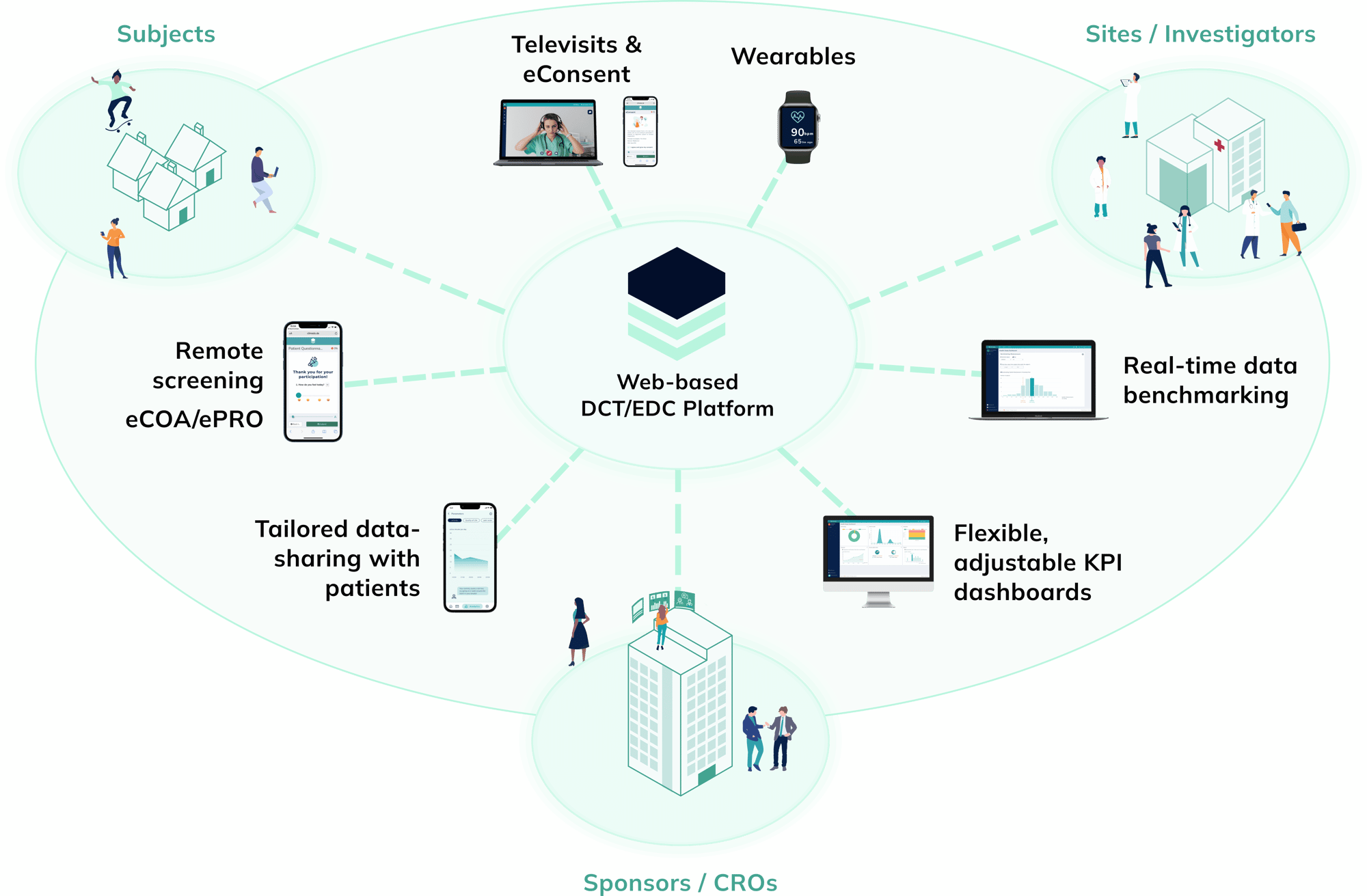
The leading European eCOA system for non-interventional studies, RWE and launch success.
Climedo offers an all-in-one eCOA and EDC solution with hybrid capabilities for non-interventional studies and real-world evidence. By using a patient-centric approach and leveraging real-time data insights and visualizations around a study’s current progress, Climedo empowers its clients to better engage with healthcare professionals and other key opinion leaders (KOLs). This boosts awareness, stimulates scientific dialogue and accelerates the launch success of new medical innovations, thus reaching more patients faster.
Founded in Munich in 2017, Climedo is a leading trusted partner for pharma, medtech, CROs and academia with over 1.7 million patients enrolled to date. Learn more at www.climedo.com.