In February 2023, we conducted a survey with Trials24 to better understand the patient perspective on clinical trials. For this, we surveyed 124 patients, 30% of whom had trial experience and 70% of whom did not, but would consider taking part in the future.
The main barriers to trial participation were a lack of communication, long travel times and difficulty in finding a suitable trial in the first place. In order to facilitate trial access, most patients believed we need to leverage more digital tools, such as wearables and telemedicine. Once enrolled, patients wanted shorter travel times and limited on-site visits, but still valued personal contact with healthcare professionals. Most patients preferred digital channels for receiving and sending important medical information. Looking at the future, over 80% with trial experience would be willing to participate again. The survey also highlighted the need for attention towards rare diseases, mental health and vulnerable communities in clinical trials.
Overall, the survey results reveal that the healthcare industry must shift towards towards more hybrid trial designs which leverage digital solutions to alleviate patient burden while maintaining human interaction and empathy in the form of communicating important information and truly listening to patient needs.
Get the survey results to learn more about:
- Current barriers and patient access to trial participation
- Expectations around travel, on-site visits & communication
- Patients' openness towards digital technologies in trials
- A look at the future and open comments
We hope you enjoy the survey results and will gain some valuable insights into the dynamic evolution of the current clinical trial landscape and what drives patient engagement. If you have any questions, please do not hesitate to reach out to us: hello@climedo.de or info@trials24.com.
CLIMEDO
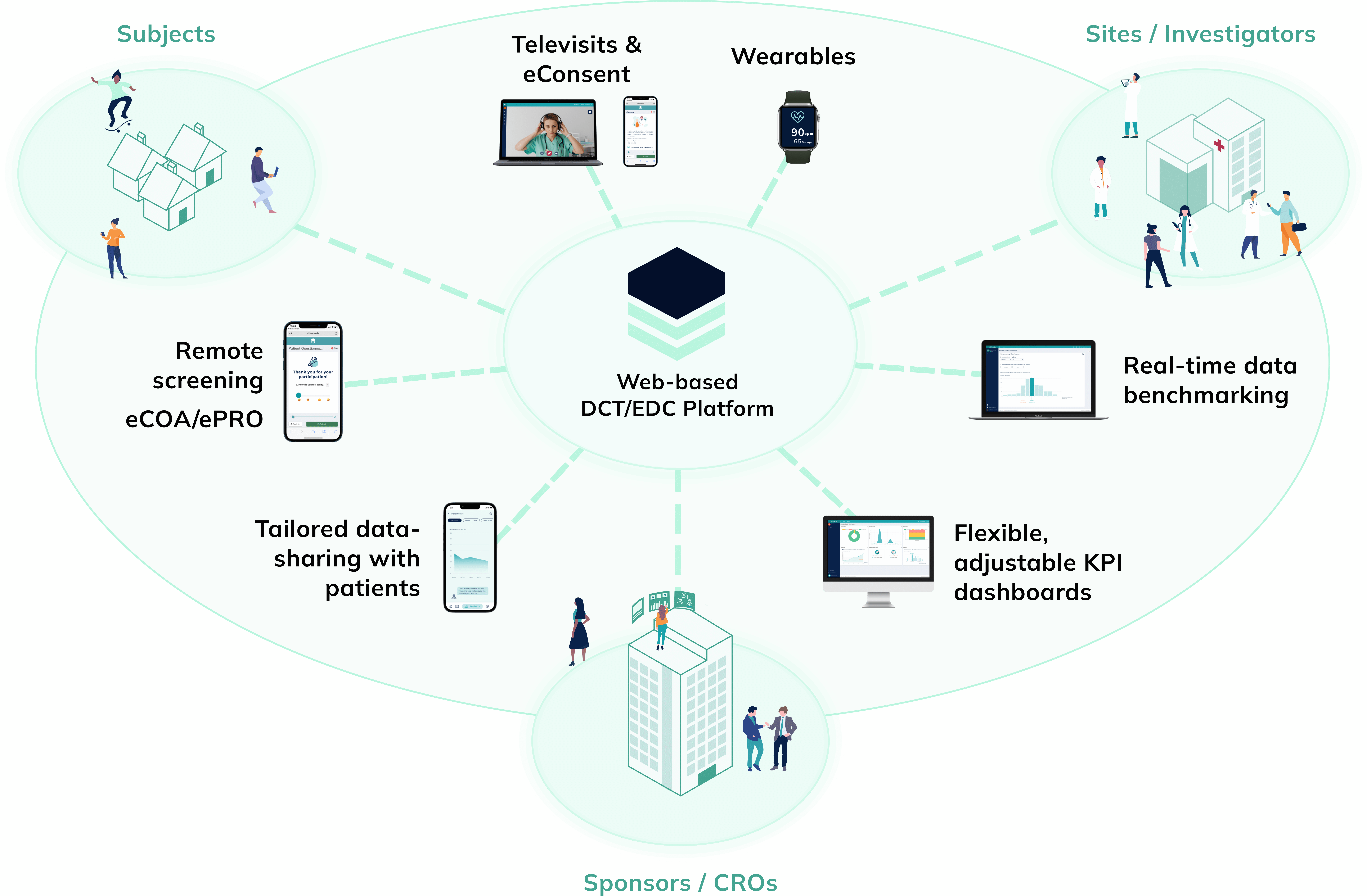
The Digital Platform for Innovative Clinical Trial Execution.
Climedo offers a software platform for hybrid clinical trials and observational studies. Its easy-to-use, modular and secure solutions for data management include electronic data capture (EDC), ePRO, eCOA, and Telemedicine. This enables pharma and medtech companies to validate their medical innovations more efficiently in the post-market phase and to capture data in decentralized, real-world settings.
As a result, they accelerate studies, save costs, and improve data flow and quality, while fostering innovative trial designs. By connecting all stakeholders (industry partners, study sites, physicians and patients) in one cloud-based system, Climedo is revolutionizing clinical research and making trials more accessible and patient centric.

TRIALS24
Accelerates Patient Recruitment
Trials24 speeds up your clinical trial’s patient recruitment by finding patients outside your site’s databases. This OutSite™ approach finds patients when the site’s databases reach their limit. Their proven end-to-end process identifies patients not registered at your clinical trial sites. Trials24 finds patients online, through patient organizations, or at their treating physicians, triple-prequalifies them, and delivers them to your site for enrollment. Trials24’s OutSite™ approach works in all clinical trial phases and for many indications – including complex ones like cancer and rare diseases.