Tuesday, May 9 2023
How do you strategically generate Real-World Evidence (RWE) at a global level and translate it into local, geographically specific evidence? What are the best practices for generating holistic evidence and integrating it into your organization's decision-making processes?
Learn more in our next Climedo Connect fireside chat on May 9 where we will discuss:
- How to strategically think about RWE generation globally and locally
- How to generate evidence in a holistic way
- Some best practices in the field of RWE from Boehringer Ingelheim
Our expert Paul Petraro, Executive Director & Global Head RWE Analytic Evidence Center at Boehringer Ingelheim, will share insights on how to consider the entire evidence generation process, from study design to data collection to analysis and interpretation, in order to generate high-quality, actionable evidence that can inform decision-making and improve patient outcomes.
Real-World Evidence refers to data that originates from real-world contexts, including electronic health records, claims data, patient registries, and various other sources. Its rising application in healthcare decision-making renders it a critical constituent of evidence-based practices. By offering an all-encompassing perspective on healthcare issues, RWE presents a compelling tool to enhance patient outcomes and influence policy decisions.
In addition to the fireside chat and Q&A with Paul, you will have the opportunity to join various live surveys, network with other attendees and, of course, ask our experts any questions you may have. We look forward to seeing you there!
Our Speakers

Paul Petraro
Executive Director & Global Head RWE Analytic Evidence Center
Boehringer Ingelheim

Laura Dosch
Account Manager
Climedo
As an Account Manager at Climedo, Laura accompanies customers from the medical device and pharmaceutical sectors on their way to the successful digitalization of clinical trials. She advises companies on the use of Climedo's modular, decentralized and patient-oriented solutions.
Agenda
-
Intro (Laura Dosch)
-
Fireside Chat with Paul Petraro: Real-World-Evidence across Borders – Why Consistency Matters
- Discussion and Wrap-up (all)
Your Takeaways
INSIGHTS
Discover how to break down barriers in Real-World Evidence and navigate global consistency.
BEST PRACTICES
Learn which RWE best practices Paul has successfully implemented at Boehringer Ingelheim and ask him any questions you may have.
NETWORKING & DISCUSSION
Talk to other industry professionals and find out how they are tackling challenges similar to yours.
CLIMEDO
The Digital Platform for Innovative Clinical Trial Execution.
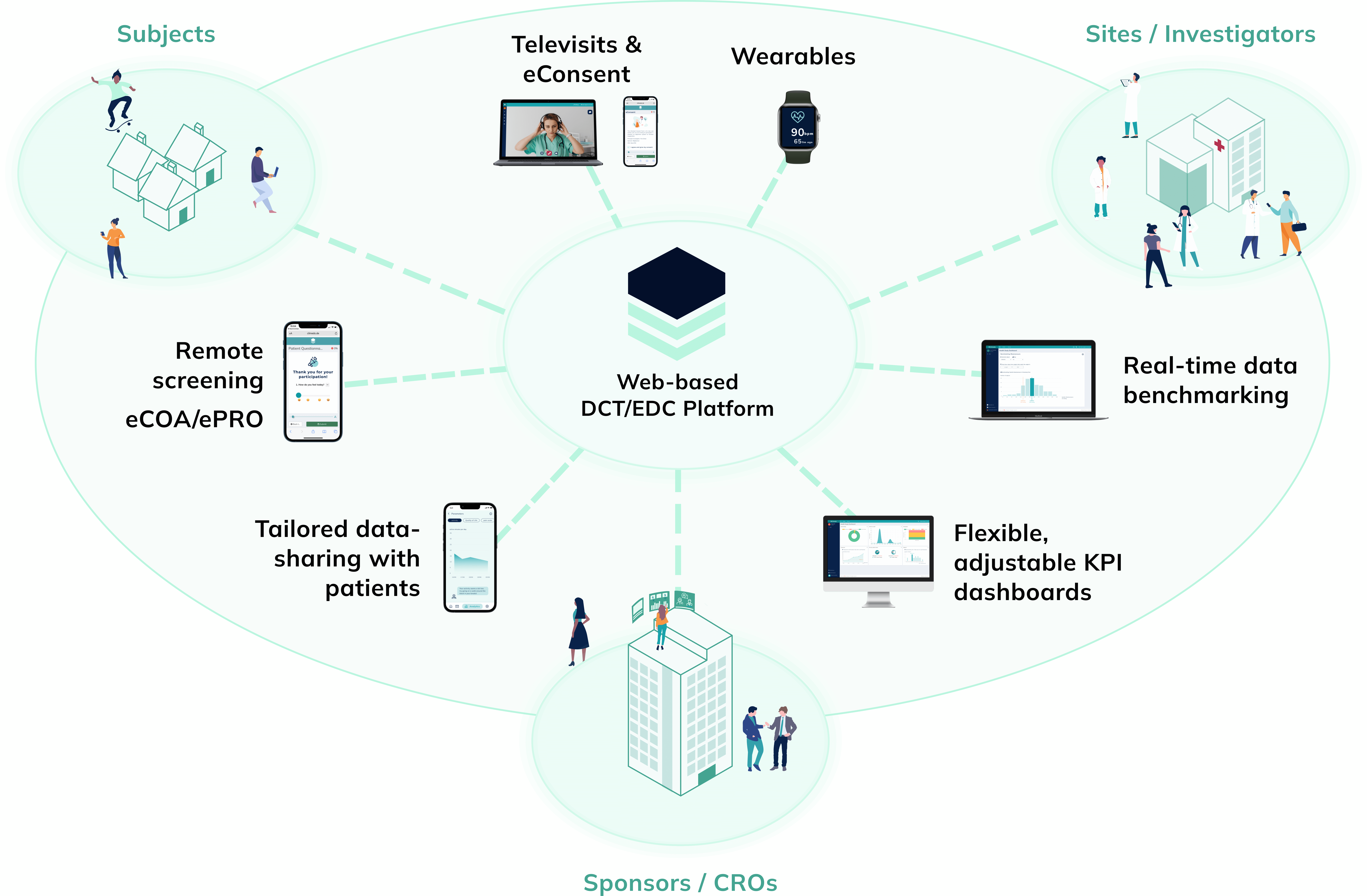
Climedo offers a digital health platform for hybrid clinical trials and observational studies. Its easy-to-use, modular and secure solutions for data management include electronic data capture (EDC), ePRO, eCOA, and Telemedicine.
This enables pharma and medtech companies to validate their medical innovations more efficiently in the post-market phase and to capture data in decentralized, real-world settings. As a result, they accelerate studies, save costs, and improve data flow and quality, while fostering innovative trial designs.
By connecting all stakeholders (industry partners, study sites, physicians and patients) in one cloud-based system, Climedo is revolutionizing clinical research and making trials more accessible and patient centric.