Whitepaper
From Data to Dialogue
Elevating Science-Based Communication in Post-Approval Studies

Swift market entry is essential for study sponsors, including pharmaceutical, biotech, and medtech companies, as well as Contract Research Organizations (CROs). While patent protection may last for 20 years, the reality is that new treatments often have only ten or eleven years of exclusivity due to product development, regulatory review and reimbursement negotiations. This leads to a race against time and exploding costs, with the average cost of developing a new drug reaching $2.3 billion in 2022.
Successful product launches depend on effective communication, especially with healthcare professionals (HCPs), such as Key Opinion Leaders (KOLs). These influential figures require not just scientific evidence but engaging discussions tailored to their expertise and interests.
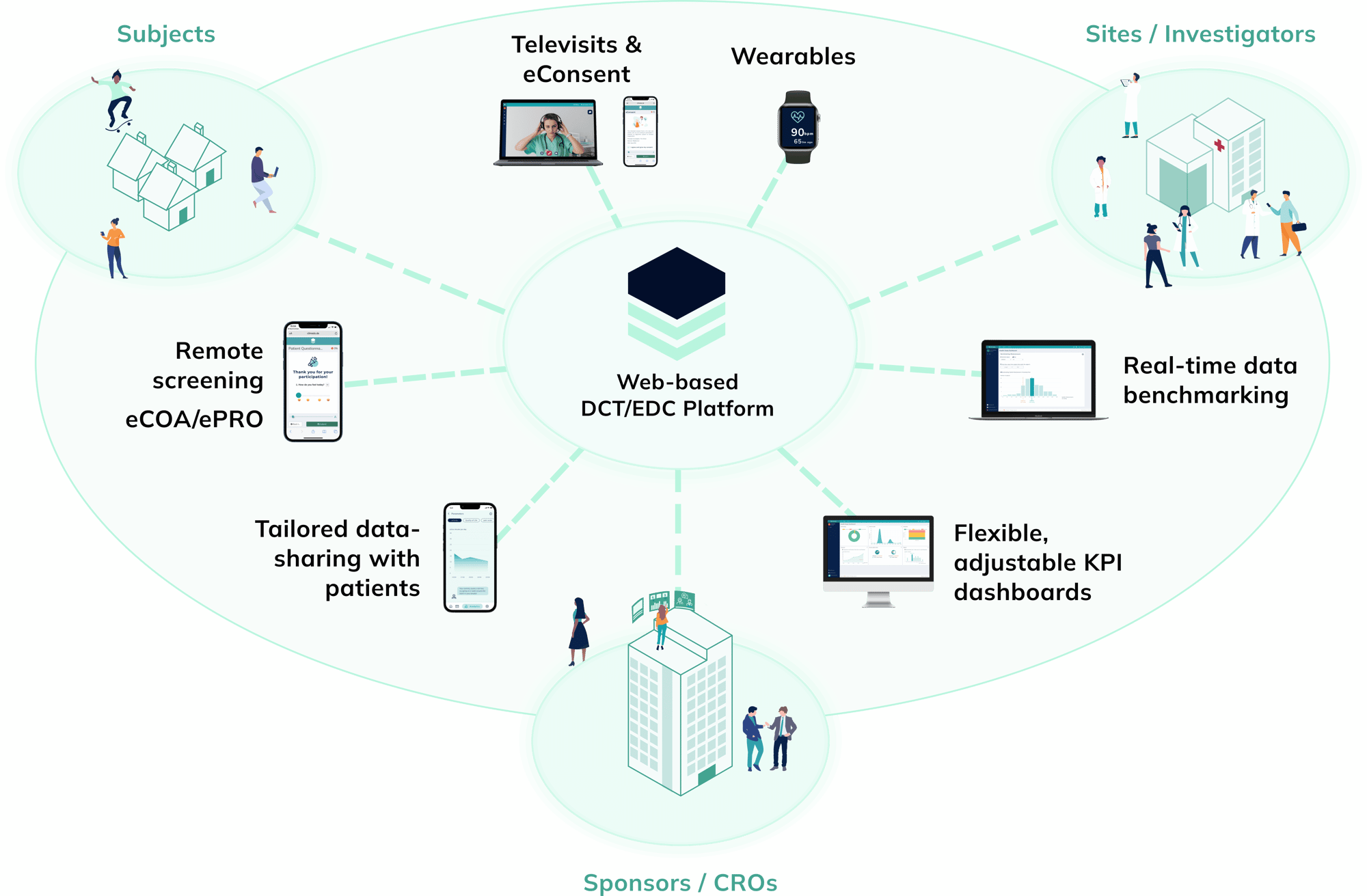
Our whitepaper, "From Data to Dialogue: Elevating Science-Based Communication in Post-Approval Studies", explores how to gain an advantage in the post-approval phase, with a focus on non-interventional studies (NIS). It outlines strategies to turn data into dialogue through modern digital solutions.
Explore the Following Topics:
- The challenges in post-approval studies (including timing, data management and patient engagement)
- How interactive digital tools can help address these challenges
- Real-world use case: Leveraging benchmarking and progression features in post-approval studies
Download our whitepaper for valuable insights and strategies to succeed in post-approval studies with real-time clinical data insights.
CLIMEDO
The leading European eCOA system for non-interventional studies, RWE and launch success.
Climedo offers an all-in-one eCOA and EDC solution with hybrid capabilities for non-interventional studies and real-world evidence. By using a patient-centric approach and leveraging real-time data insights and visualizations around a study’s current progress, Climedo empowers its clients to better engage with healthcare professionals and other key opinion leaders (KOLs). This boosts awareness, stimulates scientific dialogue and accelerates the launch success of new medical innovations, thus reaching more patients faster.
Founded in Munich in 2017, Climedo is a leading trusted partner for pharma, medtech, CROs and academia with over 1.7 million patients enrolled to date. Learn more at www.climedo.com.