Thursday June 27, 2024 | 3:00 – 4:00 PM CET
In healthcare, GenAI holds immense promise for revolutionizing medical research, diagnosis and treatment. But how can GenAI be utilized in the case of Real-World Evidence (RWE)? And to what extent can one leverage this technology effectively to enhance trial efficiency, improve data analysis capabilities and drive meaningful discoveries?
In our Climedo Connect on June 27, 2024, Dragan Mileski (Co-Founder and CTO of Climedo) explored how to meaningfully apply GenAI across the field of clinical trials, with a specific focus on RWE.
We discussed:
- Understanding GenAI: Explore the fundamentals of GenAI, including emerging trends and technology outlook, to grasp its potential in transforming RWE studies.
- Top use cases for GenAI in post-market studies: Dive into the practical applications of GenAI in post-market studies, examining its role in drug safety monitoring, comparative effectiveness analysis and more.
- Best practices for incorporating GenAI in clinical research: Learn actionable strategies and best practices for seamlessly integrating GenAI into your trials.
In addition to Dragan's presentation, attendees had the chance to gain valuable insights and contribute to the discussion on enhancing collaboration between trial physicians, sponsors, and CROs in clinical trials.
Meet Our Speakers

Bradley Fryer (Webinar Host)
Business Development Manager
Climedo
As a Business Development Manager at Climedo, Brad accompanies pharma and medtech customers on their journey to successful clinical trials in both late and post-approval phases. He advises companies on the use of Climedo's modular, hybrid and integrated EDC & eCOA/ePRO solutions to save time, boost efficiency and bring the best treatments to patients faster.

Dragan Mileski
Co-Founder and CTO
Climedo
Dragan, an IT specialist driven by entrepreneurship, co-founded Climedo in 2017 with a mission to enhance medical treatment using intelligent software solutions. As the Chief Technology Officer (CTO), he leads teams dedicated to developing cutting-edge software aimed at transforming post-market Real-World Evidence (RWE) studies, providing real-time transparency of outcomes and fostering engagement with healthcare professionals.
What was on the Agenda?
-
Intro (Bradley Fryer)
-
From Data to Insights: Leveraging GenAI in Real-World Evidence Studies (Dragan Mileski)
-
Discussion and networking (all)
- Wrap-up (Bradley Fryer)
Your Takeaways
INSIGHTS
Valuable insights into the top use cases of GenAI, emerging trends and its potential impact in clinical trials.
BEST PRACTICES
Discover practical applications for incorporating GenAI into your clinical trials, ensuring smooth integration and enhanced trial outcomes.
NETWORKING & DISCUSSION
Talking to other industry professionals and finding out how they are tackling challenges similar to yours.
CLIMEDO
The leading European eCOA system for non-interventional studies, RWE and launch success
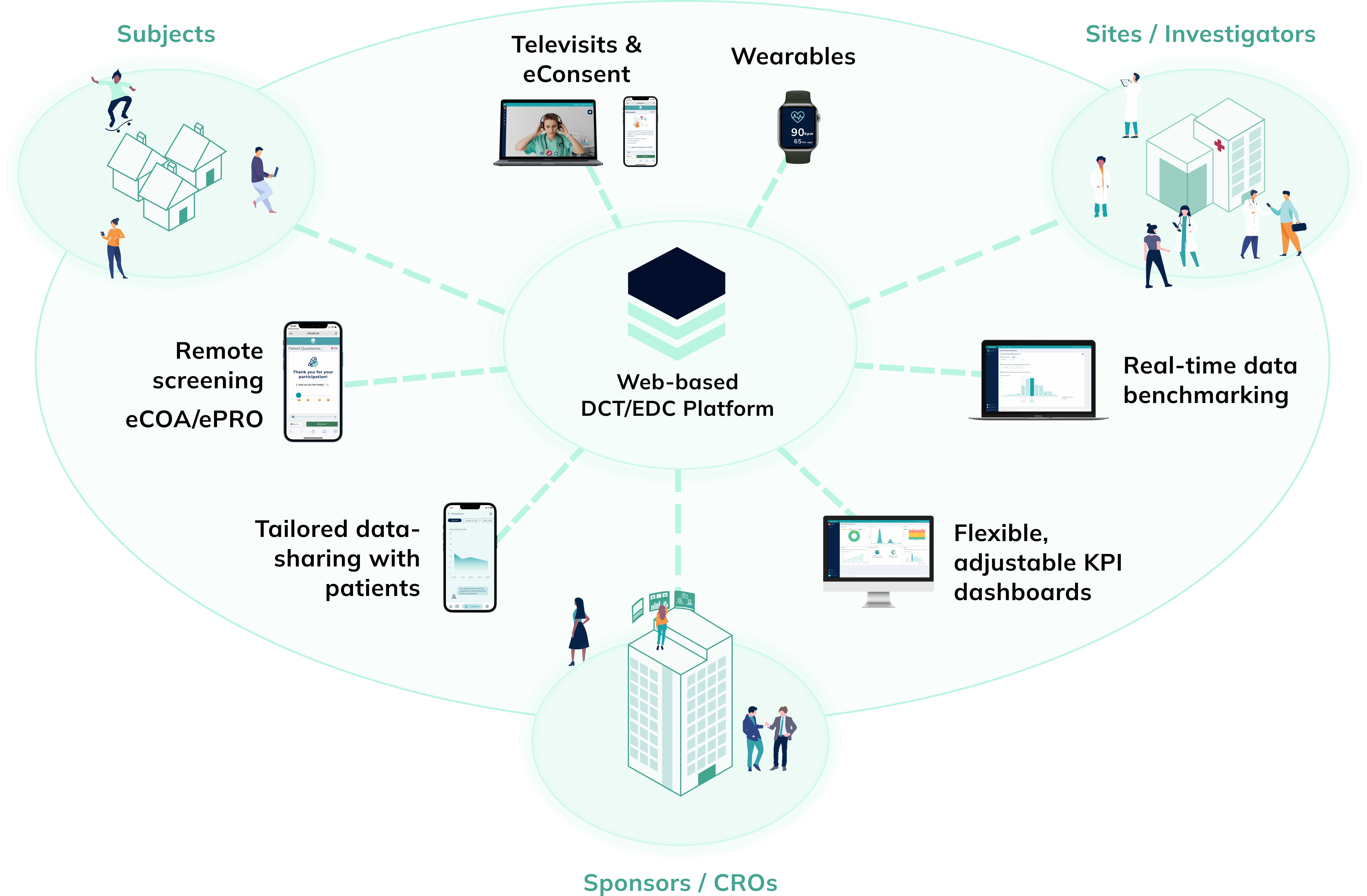
Climedo offers an all-in-one eCOA and EDC solution with hybrid capabilities for non-interventional studies and real-world evidence. By using a patient-centric approach and leveraging real-time data insights and visualizations around a study’s current progress, Climedo empowers its clients to better engage with healthcare professionals and other key opinion leaders (KOLs). This boosts awareness, stimulates scientific dialogue and accelerates the launch success of new medical innovations, thus reaching more patients faster.
Founded in Munich in 2017, Climedo is a leading trusted partner for pharma, medtech, CROs and academia with over 1.7 million patients enrolled to date. Learn more at www.climedo.com.