Real-world evidence (RWE) offers perspectives on the clinical efficacy and cost of treatments, the performance of healthcare systems, highlighting unmet needs and optimal clinical practices in real-world settings. But how can RWE be leveraged to better understand new oncology mutations and patient characteristics, optimise treatment pathways and improve clinical outcomes? And how can we generate evidence to redefine the current Standard of Care?
In our Climedo Connect on January 25, 2024, Dr. Helene Vioix (Director Global Value Development Oncology, Merck) delved into best practices on how to maximise the impact of real-world data to best engage Healthcare Professionals (HCPs) and Key Thought Leaders (KTLs). We discussed:
- Engaging Key Thought Leaders (KTLs) throughout the feasibility phase for invaluable insights.
- Creating novel evidence and gaining Health Technology Assessment (HTA) acceptance through innovative methodologies.
- Leveraging KTL support to disseminate results to wider Key Opinion Leader (KOL) groups.
- Exploring an innovative approach that supports the enhancement of more traditional registries, all while navigating regulatory challenges.
In addition to Helene's presentation, attendees had the chance to take part in live polls, ask any questions and network with like-minded professionals.
Meet Our Speakers

Laura Dosch (Webinar Host)
Account Manager
Climedo
As an Account Manager at Climedo, Laura accompanies pharma and medtech customers on their journey to successful clinical trials in the post-approval phase. She advises companies on the use of Climedo's modular, hybrid and integrated EDC & eCOA/ePRO solutions to save time, boost efficiency and bring the best treatments to patients faster.

Dr. Helene Vioix
Director Global Value Development Oncology
Merck
Helene, a distinguished leader in HEOR & RWE, stands out for her global impact and collaborative approach. With a portfolio over 50 publications, she showcases a deep understanding of payer needs and compliance in pharma. Her commitment to impactful healthcare solutions is evident in her collaborative spirit, unique initiatives and strategic acumen, making her an invaluable asset to cross-functional projects with a far-reaching influence.
What was on the Agenda?
-
Intro (Laura Dosch)
-
Unlocking RWE’s Potential for HCP Engagement (Dr. Helene Vioix)
-
Discussion and networking (all)
- Wrap-up (Laura Dosch)
Takeaways
INSIGHTS
Gain insights into the evolving landscape of RWE strategies for optimal HCP engagement.
BEST PRACTICES
Discover effective strategies for creating novel evidence and gaining Health Technology Assessment acceptance.NETWORKING & DISCUSSION
Talk to other industry professionals and find out how they are tackling challenges similar to yours.
CLIMEDO
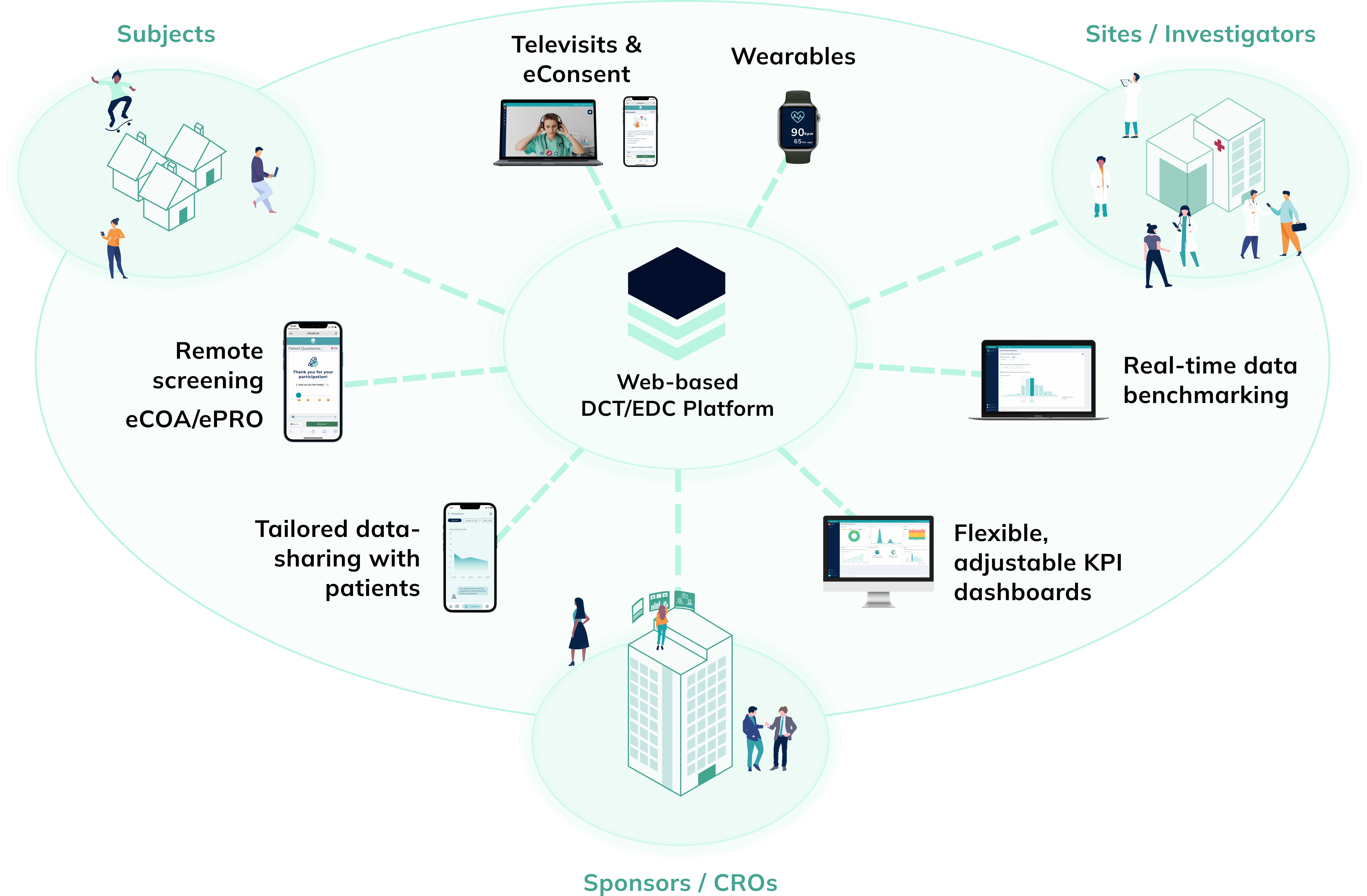
The leading European eCOA system for non-interventional studies, RWE and launch success
Climedo offers an all-in-one eCOA and EDC solution with hybrid capabilities for non-interventional studies and real-world evidence. By using a patient-centric approach and leveraging real-time data insights and visualizations around a study’s current progress, Climedo empowers its clients to better engage with healthcare professionals and other key opinion leaders (KOLs). This boosts awareness, stimulates scientific dialogue and accelerates the launch success of new medical innovations, thus reaching more patients faster.
Founded in Munich in 2017, Climedo is a leading trusted partner for pharma, medtech, CROs and academia with over 1.7 million patients enrolled to date. Learn more at www.climedo.com.